Greek patients with Cystic Fibrosis (CF) are registered in:
- the European Cystic Fibrosis Society Patient Registry (ECFSPR) of the European Cystic Fibrosis Society (ECFS)
- in the National Registry of Cystic Fibrosis Patients of our country of the Ministry of Health
Patients’ Registries collect demographic and clinical data, which is pseudonymised and anonymised.
Patients’ registries are useful and valuable tools that contribute to better patient care with the ultimate goal of improving patients’ health .
Moreover, Patients’ Registries give the chance to patients to take part in clinical trials of new medicines.
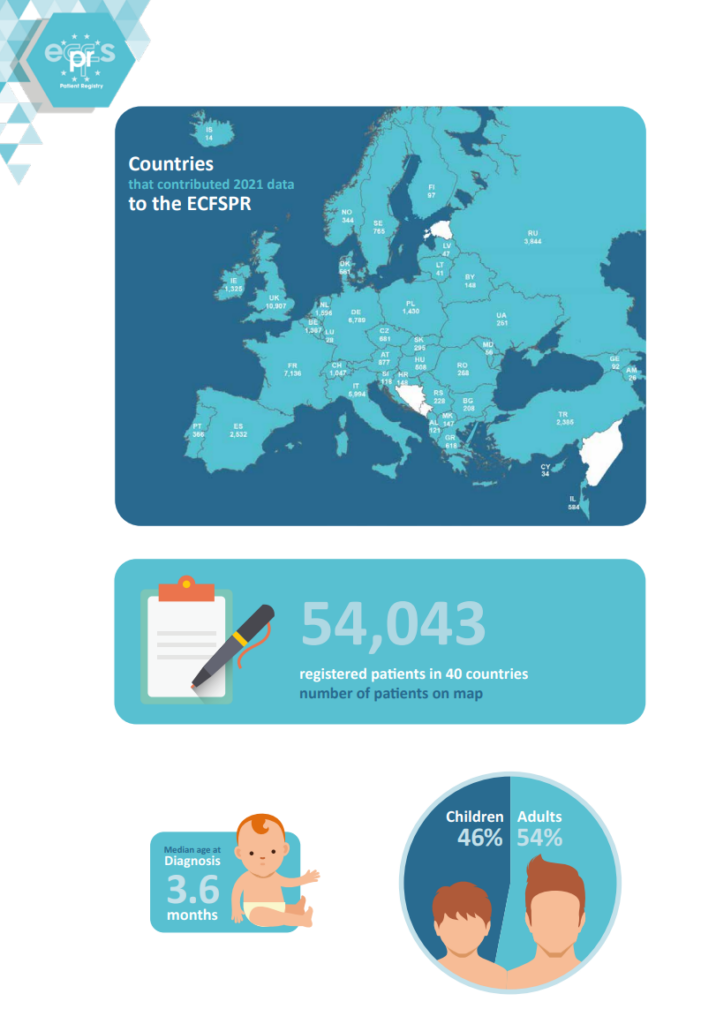

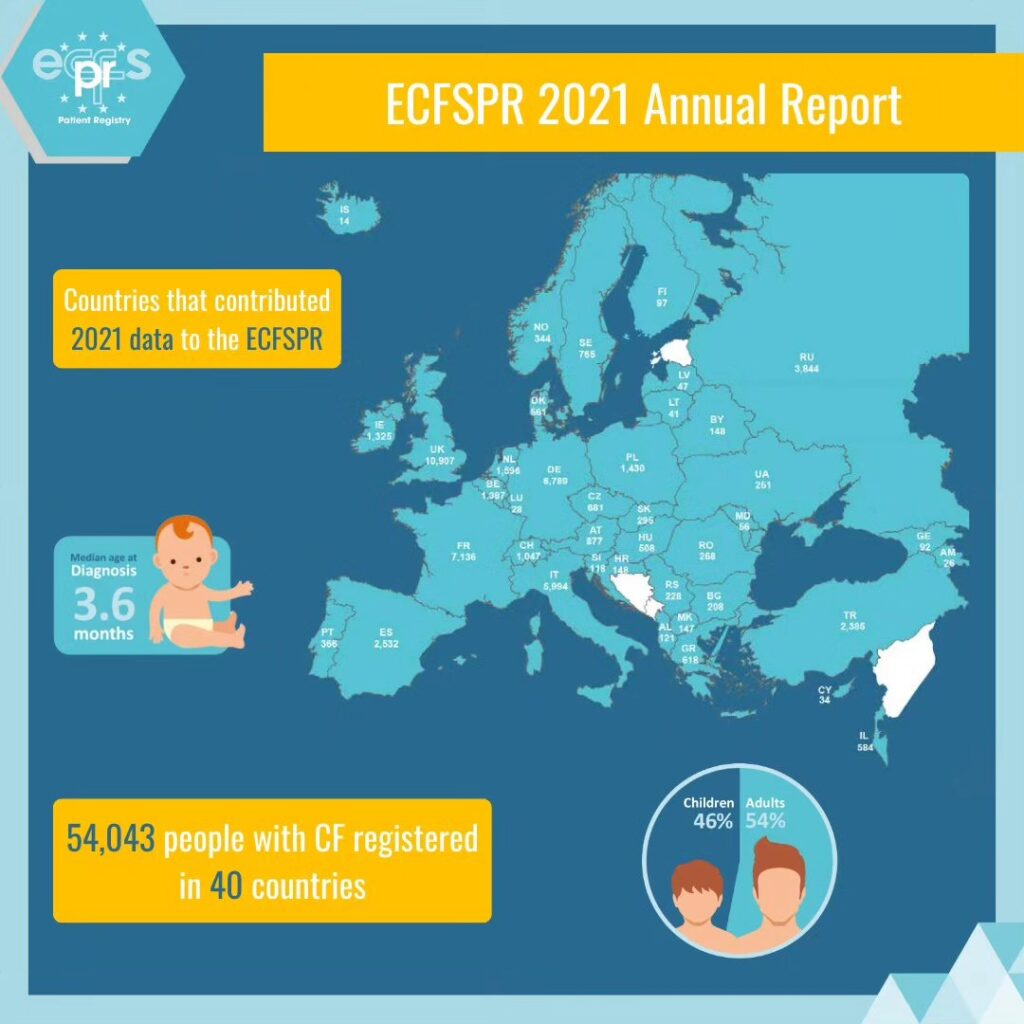
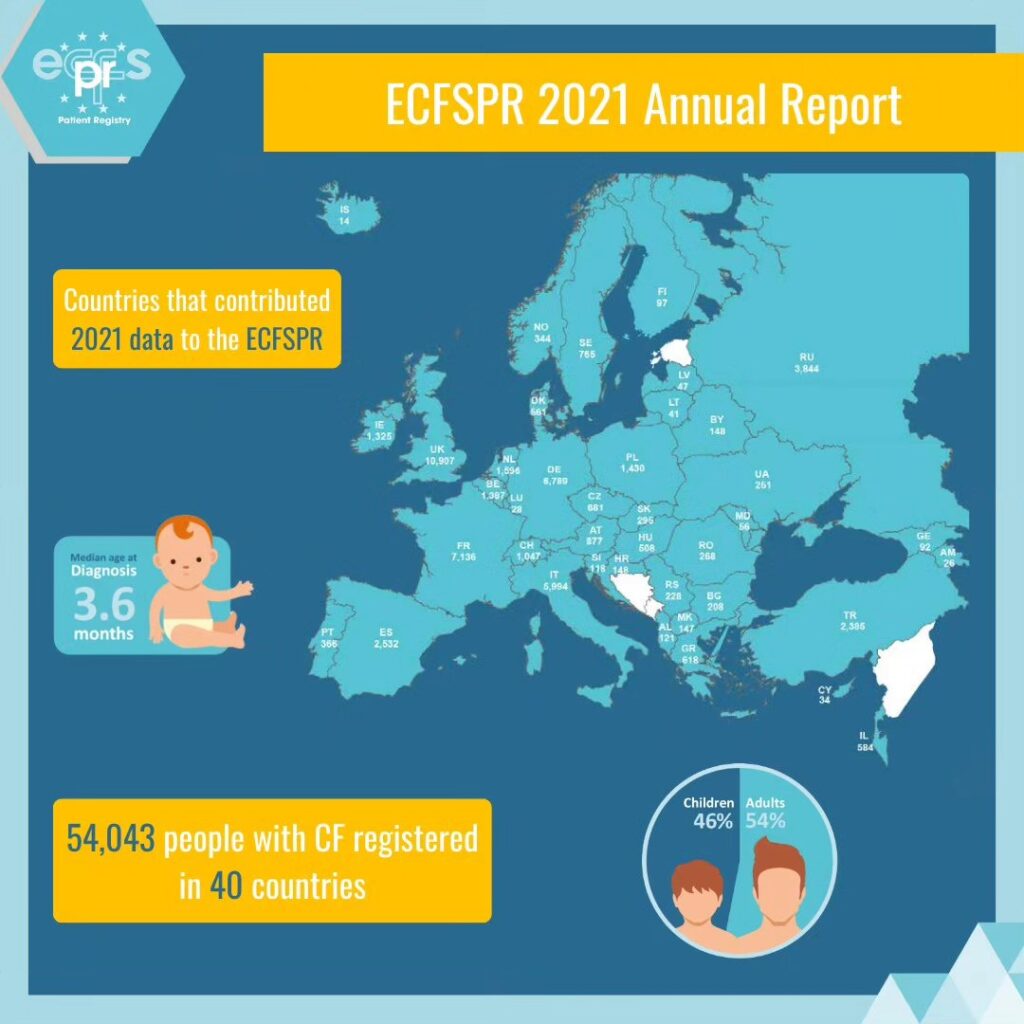
European Cystic Fibrosis Society Patient Registry- ECFSPR
The European Cystic Fibrosis Society Patient Registry ECFSPR- European Cystic Fibrosis Society Patient Registry was created in 2008 by the European Cystic Fibrosis Society- ECFS. The ECFSPR is linked to the National CF Patients’ Registry and collects pseudonymised demographic and clinical data from more than 54,000 CF patients from 40 participating European countries. The ECFSPR reflects the situation of Cystic Fibrosis across Europe. Read more here.
In 2010, the CF children Center of Thessaloniki in the General Hospital “Hippocratio” was the first Greek CF Center that joined the ECFSPR. After many efforts in continuous collaboration of the Association and the CF Scientific Committee of our country, we managed for the first time in 2015 to include in the ECFSPR all the Greek Cystic Fibrosis Centres in Thessaloniki and Athens. The participation of our country in the ECFSPR increased the credibility and dynamics of Greece. Also in this way Greece contributed to the joint efforts of the European Cystic Fibrosis Society to register patients.
The data collected by each CF Centre in our country is pseudonymised and sent once a year to the ECFSPR. Data collection is done with the consent of the patients or their parents in the case of minor patients. More information in the updated Information and consent form.
The information collected by the ECFS is used to measure, research and compare aspects of Cystic Fibrosis and its treatment in participating countries, to deepen understanding of the disease, to improve standards of care, to provide data for epidemiological research and to facilitate public health planning.
Benefits of Greece's participation in the ECFSPR
- Evaluating the effectiveness of various treatments of each CF Centre and comparing them with those of other CF Centres in other countries in Europe and around the world.
- Assessing which treatments are most useful and whether they improve patient care.
- Assessment of the epidemiology of the disease, e.g. complications, new infectious strains.
- Designing future innovative medicines for CF patients.
- Identifying groups of patients eligible for new treatments, ensuring access to innovative therapies.
- Apart from the scientific community, the data of the ECFSPR is also used by patient representatives for the planning and documentation of the Association’s positions and demands in order to improve the health and quality of life of patients.
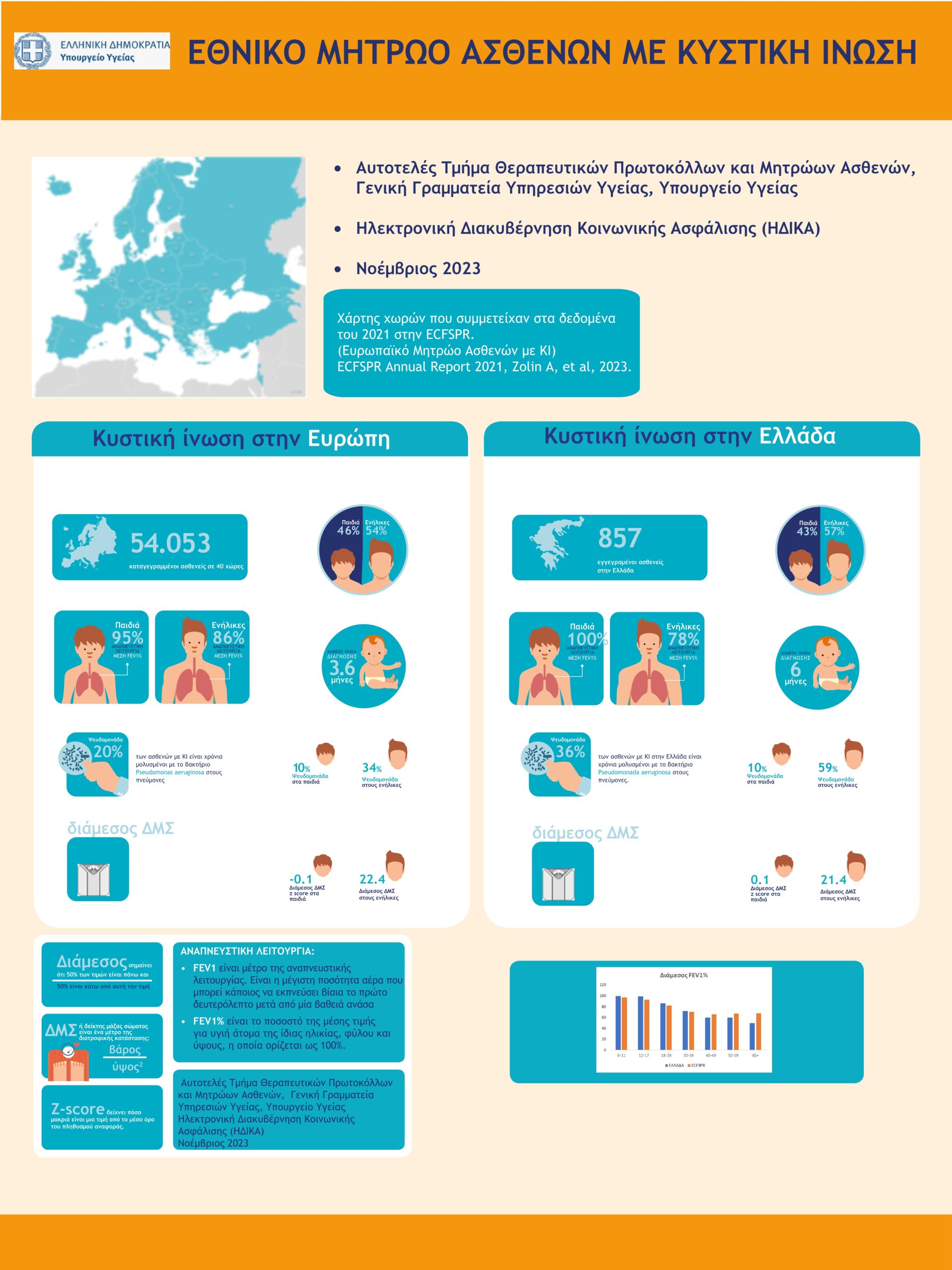
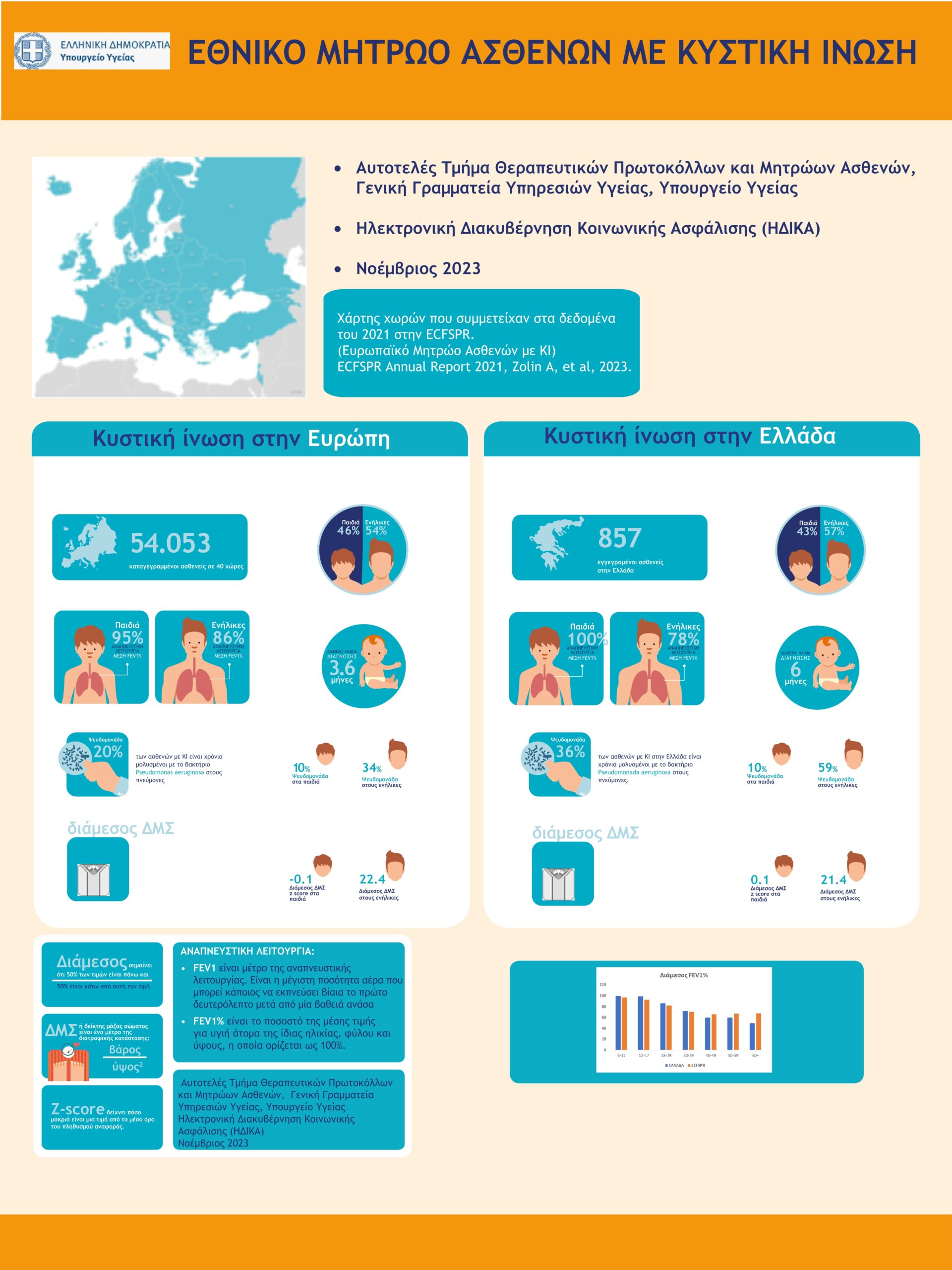
National Cystic Fibrosis Patients' Registry
The National Cystic Fibrosis Patients’ Registry of Greece was established in 2020 by the Greek Ministry of Health (here the decision).
It is one of the first patient registries in Greece and is linked to the European Cystic Fibrosis Registry ECFSPR.
It isa key tool for the provision of Health Services to Greek patients with CF.The establishment of the National CF Patients’ Registry was necessaryfor the completion of the reimbursement negotiation procedures for CFTR Modulators treatments in Greece.
The registration of Greek patients with Cystic Fibrosis in the National Registry is mandatory based on a decision of the Greek Ministry of Health (here).
Responsible for carrying out the processing and storage of the data of patients in the National CF Patients’s Registry, on behalf of Ministry of Health, is IDIKA
The management and supervision of the Registry is carried out by the Independent Department of Treatment Protocols and Patient Registries of the Greek Ministry of Health.
The Data Protection Officer (DPO) of the Ministry of Health, as the controller, monitors the compliance of the establishment and operation of the Registry for the protection of patients’ personal data in cooperation with the DPO of IDIKA SA.
The monitoring, processing and periodic updating of the National Register is carried out by the Working Group, which consists of members of the Scientific Committee and a patient representative of the Association.
The first infographics in Greece with data of Greek patients from the National Cystic Fibrosis Registry was published in December 2023 by the Ministry of Health (
here
).
Working Group Members of the National CF Patients' Registry
- TSANAKAS IOANNIS, Professor Emeritus of Pediatric Pulmonology, Aristotle University of Thessaloniki, Aristotle University of Thessaloniki, AUTH
- DIAMANTEA FILIA, Pulmonologist, Director of the Cystic Fibrosis Unit for Adults, “SISMANOGLEIO” General Hospital
- DUROS KONSTANTINOS, Professor of Pediatrics – Pediatric Pulmonology, 3rd Pediatric Clinic of the University of Athens, P.G.H. “ATTIKON”
- THIRAIOS ELEITHERIOS, General Practitioner/Family Physician, Director of H.S.Y., Head of the General Directorate of O.D.I.P.Y. S.A., Secretary General of the Athens Medical Society
- KARETSI ELENI, Pulmonologist, Director of E.S.Y., University General Hospital of Larissa
- LOUKOU IOANNA, Pediatrician, Director of the Cystic Fibrosis Department, Athens Children’s Hospital “Aghia Sophia”
- MANIKA AIKATERINI, Associate Professor of Pulmonology – Tuberculosis, University of Thessaloniki, Adult Cystic Fibrosis Centre, Department of Pulmonology, University of Thessaloniki, General Hospital “G. PAPANIKOLAOU”
- PANAGIOTA MITROU, Specialist Pathologist – Diabetologist, Doctor of the University of Athens, Head of the Independent Department of Treatment Protocols and Patient Registers of the Ministry of Health
- BOLIS KONSTANTINOS, Pediatrician, Coordinating Director of the Pediatric Department, University Pediatric Clinic, Pediatric Pulmonology Department, University General Hospital of Patras “PANAGIA I VOTHIEIA”
- PARASKAKIS EMMANOUL, Associate Professor of Pediatrics, Head of the Pediatric Pulmonology Unit, Pediatric Clinic of PAGNI, President of the Hellenic Pediatric Pulmonology Society
- PITSIDIANAKIS GEORGIOS, Pulmonologist – Tuberculologist, Director of the Cystic Fibrosis Clinic for Adults and Bronchiectasis, Pulmonology Clinic, Heraklion University General Hospital (PAGNI)
- SPINOU ANNA, President of the Panhellenic Cystic Fibrosis Association, Member of the Board of Eurordis- Rare Diseases Europe, European Patients’ Academy (EUPATI) fellow
- FOTOULAKI MARIA, Professor of Pediatrics – Pediatric Gastroenterology, Director of the 4th University Pediatric Clinic of the Aristotle University of Thessaloniki, “PAPAGEORGIOU” Hospital, Head of the Cystic Fibrosis Unit of the Intersectoral Cystic Fibrosis Center of the Aristotle University of Thessaloniki.
- HATZIAGOROU ELPIDA, Professor of Pediatrics – Pediatric Pulmonology, Aristotle University of Thessaloniki, Head of the Cystic Fibrosis Unit, 3rd Pediatric Clinic, GP “IPOKRATEIO”
The coordinator of the Working Group is TΣANAKAS IOANNIS.
