4th Health Innovation Conference:
The Importance of Patient Participation in Health Technology Evaluation
“Medical Innovation versus COVID-19”
Dimitris Kontopidis, representing patients, as Vice President of the Hellenic Patients’ Association and Honorary President of the Hellenic Cystic Fibrosis Association, participated in the 4th Health Innovation Conference held online on Tuesday 16 June 2020, moderated by journalist Emilio Negi.
Round Table I : “EVALUATION OF HEALTH TECHNOLOGIES”
Vassilis Kontozamanis, Deputy Minister of Health
Dimitris Kontopidis, Vice President of the Hellenic Patients’ Association, Honorary President of the Hellenic Cystic Fibrosis Association
Elena Houliara, President and CEO AstraZeneca Greece & Cyprus, President of PhRMA Innovation Forum
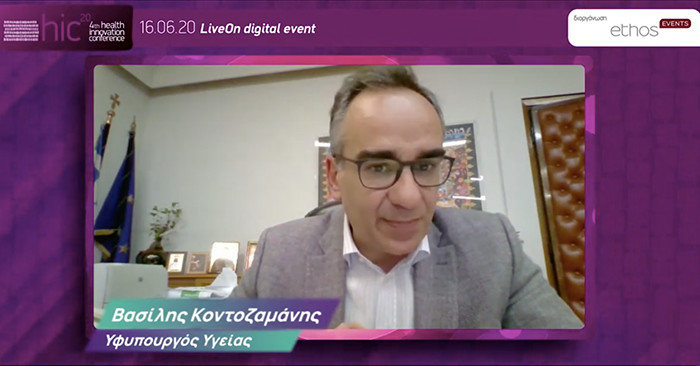

In the first panel of the Conference, which dealt with the issues of innovation and evaluation of medicines, the Deputy Minister of Health, Mr.Vassilis Kontozamanis, stressed the importance of creating an HTA Evaluation Agency, as he admitted that the Evaluation and Negotiation Committees suffer from understaffing. At the same time, he recalled that last September corrections were made to distortions in the HTA Evaluation and Negotiation Committees. The Deputy Minister noted that in cases of emergency, the issue of cost was overcome and patients had access to innovative treatments that can blow a budget. Finally, he maintained that there would be structural measures on clawback to rationalise the system to ensure the viability of EOPY and pharmaceutical companies.
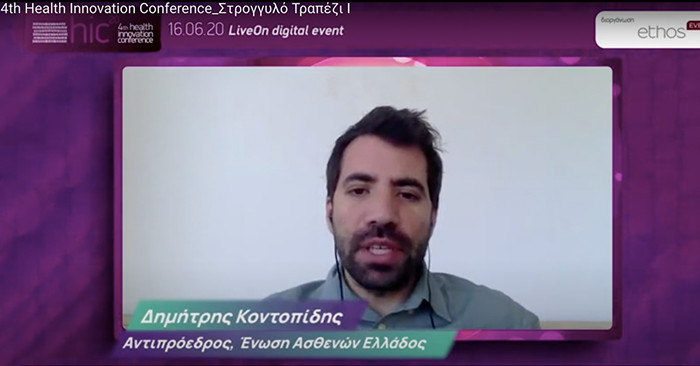

Dimitris Kontopidis from the patients’ side,
made it a priority to involve patients in processes that will help to highlight the real costs of existing treatments and the benefits of innovative and theoretically expensive treatments. At the same time, it raised the issues of timing and transparency of the procedures, as well as the issue of equality between Greeks and other European patients in terms of access to treatments. At the conclusion of the panel discussion he concluded “..Now with the new institutional body of the Hellenic Patients Association we express our availability as valuable allies and our request for our representation both in the Health Technology Assessment Committees and in the Patient Registries. We believe that the government is promoting a patient-centred system and democracy in healthcare, and the speed of approval of innovative treatments concerns all of us, it concerns costs and lives.”
Throughout the discussion, he referred extensively to the topical negotiation of the breakthrough treatment “Trikafta” as a case study process for evaluating an innovative drug.
He began by seeking a definition of what an innovative drug is, what it can really offer patients, the importance of speed of reimbursement approval and the importance of patients in the process of its evaluation.
The stages of innovation in Cystic Fibrosis
Dimitris Kontopidis went on to say that “Cystic Fibrosis patients, once upon a time – not too long ago – but in my own adolescence, were lost in the 20-25 age range until recently. Now, for children born today, we can and do talk about a life expectancy of 60 years with the innovative therapies that have been developed so far. The journey of innovation in medicine has gone through several stages in Cystic Fibrosis.
In the beginning, we went from minimal intravenous antibiotics to a variety of inhaled antibiotics. In the next stage we moved to inhaled “pocket” antibiotics. Instead of patients wasting 20 minutes for each inhaled antibiotic through a special nebulizer, they could now carry it with them and do it in one spray, saving time but also improving their compliance to treatment.
We are now in the era where we have moved from antibiotics that target the symptoms of the disease, to personalised innovative treatments per patient mutation that target the causes of the disease. One such revolutionary treatment is ‘Trikafta’, which was approved for marketing in America last November and is expected to be approved in the coming months by the European Medicines Agency. The magnitude of the revolution in the effectiveness of the drug can be illustrated by the following example. We see in America patients who were in end-stage respiratory failure, on permanent oxygen supply and on a lung transplant list, coming off the list and not needing oxygen supply within a few weeks of taking this miracle pill, because it is a pill.
Access to an innovative treatment
What is important, however, is the timely reimbursement of such an innovative drug as ‘Trikafta’. So when a list price – if we could call it that – of USD 300 000 per patient per year in America is announced – the questions arise as to whether such a cost is considered expensive and, above all, whether it is worth being reimbursed by the Greek state. This is where the Commission for the Evaluation of Health Technologies is called upon to provide answers, with indicators of effectiveness in relation to costs and, of course, patient participation.”
The value of patient participation in the evaluation
In response to the report of the Deputy Minister of Health, Mr. Kontozamanis, that some treatments can blow the state budget, Dimitris Kontopidis said that by personally measuring how much he personally “costs” as a patient to the state, he concluded that his monthly treatments cost several thousand euros in pharmaceutical costs. If one takes into account the cost of hospitalization, the additional tests needed on a regular basis, along with the inability of both the patient and his caregivers to work full-time, one understands that these costs can be very close to the cost of a seemingly expensive drug. This is the value of involving patients in the evaluation of a truly innovative drug and not just a new drug since many benefits are not captured by clinical data alone.
At the same time, in the context of the research and development of orphan drugs, pharmaceutical companies should continue to be given incentives so that we reach the happy event, as in the case of Cystic Fibrosis, of discovering such treatments. The speed of approval and reimbursement of such drugs is also the necessary incentive for continued research into therapies that literally save lives, with the aim of getting them into the hands of every patient in time and without inequalities.
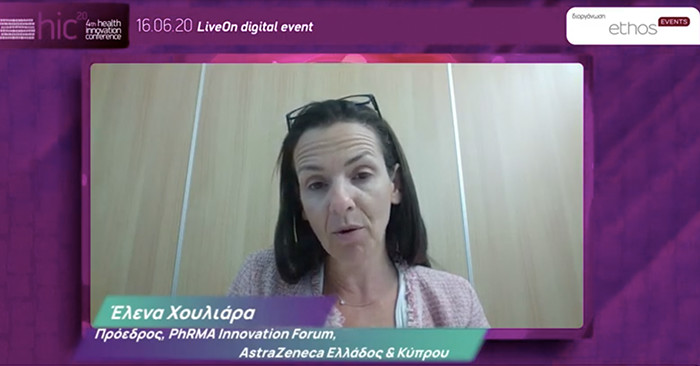

On the part of the pharmaceutical industry
Mrs. Houliara, President and CEO of AstraZeneca Greece & Cyprus and President of the PhRMA Innovation Forum, first of all congratulated Dimitris Kontopidis for the moving demonstration of the value of innovation. He then went on to mention the good news about the company’s collaboration with the University of Oxford, highlighting that 2 billion doses of COVID-19 vaccine have been secured, 400 million of which will be for Europe. He also recalled that 172 approvals have been given by the European agency and only 69 of these drugs are reimbursed in our country, stressing that in the last two years since the competent committee was launched, the new drugs that have been given the green light can be counted on the fingers of one hand.
You can watch the video of the debate here.





